J. Am. Chem. Soc 2008
Modular Fluorescent Benzobis(imidazolium) Salts: Syntheses, Photophysical Analyses, and Applications
Andrew J. Boydston, Peter D. Vu, Olga L. Dykhno, Vicki Chang, Alvin R. Wyatt, II, Adam S. Stockett, Eric T. Ritschdorff, Jason B. Shear, and Christopher W. Bielawski
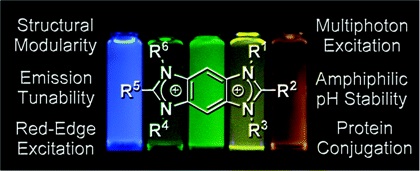
A series of benzobis(imidazolium) (BBI) salts has been prepared and studied as a new class of versatile fluorescent materials. Using a high yielding, modular synthetic strategy, BBI salts with a range of functionality poised for investigating fundamental and applications-oriented characteristics, including emission wavelength tunability, solvatochromism, red-edge excitation, chemical stability, multiphoton excitation, and protein conjugation, were prepared in overall yields of 40-97%. Through structural variation, the BBIs exhibited em ranging between 329 and 561 nm while displaying fs up to 0.91. In addition, the emission characteristics of these salts were found to exhibit strong solvent dependencies with Stokes shifts ranging from 4570 to 13 793 cm-1, depending on the nature of the BBI core. Although red-edge effects for BBI salts with Br and BF4 counterions were found to be similar, unique characteristics were displayed by an analogue with MeSO4 anions. The stability of an amphiphilic BBI was quantified in aqueous solutions of varying pH, and >85% of the emission intensity was retained after 2 h at pH 3-9. Through multiphoton excitation experiments in aqueous solutions, a BBI salt was found to exhibit three-photon fluorescence action cross sections similar to serotonin. The application of BBI salts as fluorescent protein tags was demonstrated by conjugating bovine serum albumin to a maleimide-functionalized derivative.