Fabrication of Biological Materials Subcellular Dosing
High Resolution Spatio-Temporal Dosing of Subcellular Targets
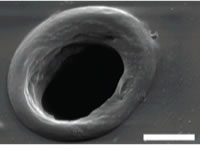
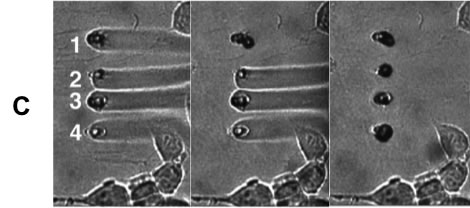
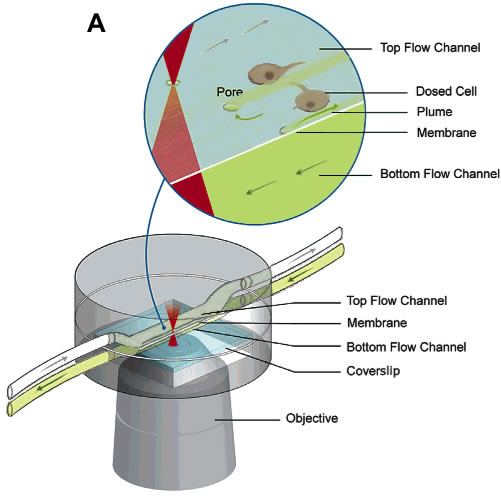 (A) A laminar flow device used for parallel dosing of cellular targets with desired chemical species. This approach is based on laser-mediated ablation of polymer membranes that serve as substrates for cell attachment, and to separate two stacked flow chambers: a cell medium and a dosant chamber. Aperture creation introduces laminar flow streams from the high pressure dosant chamber into the cell chamber allowing for specific cellular features to be dosed (B) SEM of characteristic pores in 2.5-µm thick Mylar membrane and 7.5-µm thick polyimide films (C) Reagent plumes streaming into a cell field that are sequentially stopped by fabrication of protein plugs over selected pores.
(A) A laminar flow device used for parallel dosing of cellular targets with desired chemical species. This approach is based on laser-mediated ablation of polymer membranes that serve as substrates for cell attachment, and to separate two stacked flow chambers: a cell medium and a dosant chamber. Aperture creation introduces laminar flow streams from the high pressure dosant chamber into the cell chamber allowing for specific cellular features to be dosed (B) SEM of characteristic pores in 2.5-µm thick Mylar membrane and 7.5-µm thick polyimide films (C) Reagent plumes streaming into a cell field that are sequentially stopped by fabrication of protein plugs over selected pores.![]()
Multi-directional Stream Control
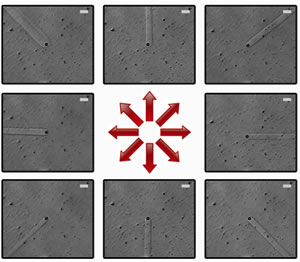
To further increase the spatio-temporal resolution of our cell-dosing device, we have replaced the simple flow cell containing the cell medium with a multiple outlet asterisk flow cell. In conjunction with computer-activated pinch valve switching, the stream orientation can be changed on-the-fly, which allows for greater accessibility of sites of interest, and also permits brief exposures of cells to desired reagents. Eight primary flow directions can be obtained, which can be increased to sixteen directions by opening two valves simultaneously. Sub-second resolutions can be obtained. Scale bars, 20 µm.
Neutrophil Polarization in Response to fMLP
Movie showing neutrophil polarization in response to fMLP. A reagent stream consisting of 0.5 µM fMLP with 3% BSA was introduced into the cell chamber via a pore formed in Mylar membrane.
Individual pores can be formed upstream from a cell process that results in a plume directly intersecting only a single cell process. This movie shows the guidance of a dHL-60 cell through ca. 90 deg in a clockwise arc by changing the orientation of a stream of 100 nM fMLP through a series of six steps. In this experiment, stream orientations were changed in increments smaller than 45 deg. For each of the six dHL-60 cells steered in this manner, cells continuously reinforced their polarity (and thus, movement) toward the gradient established by the chemoattractant streams.
